Presented by: Miltos Balidis, MD, PhD, FEBOphth, ICOphth
Edited by: Penelope Burle de Politis, MD
A 70-year-old woman was referred for diagnosis and management of persistent ocular surface inflammation in both eyes.
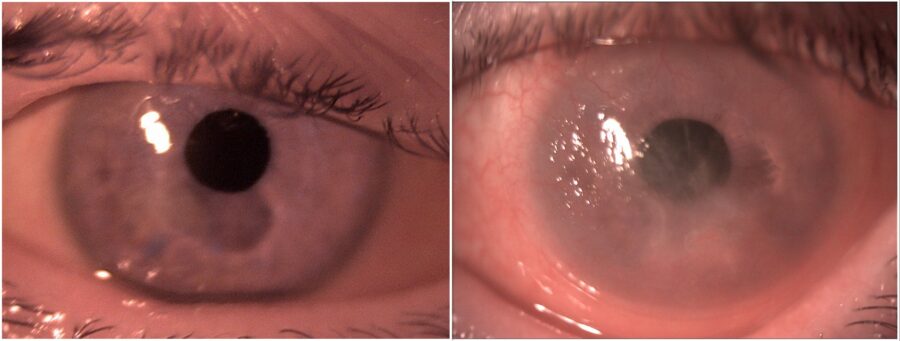
Figure 1: Slit-lamp photograph showing bilateral conjunctival inflammation and corneal scarring, more pronounced in the left eye.
Case History
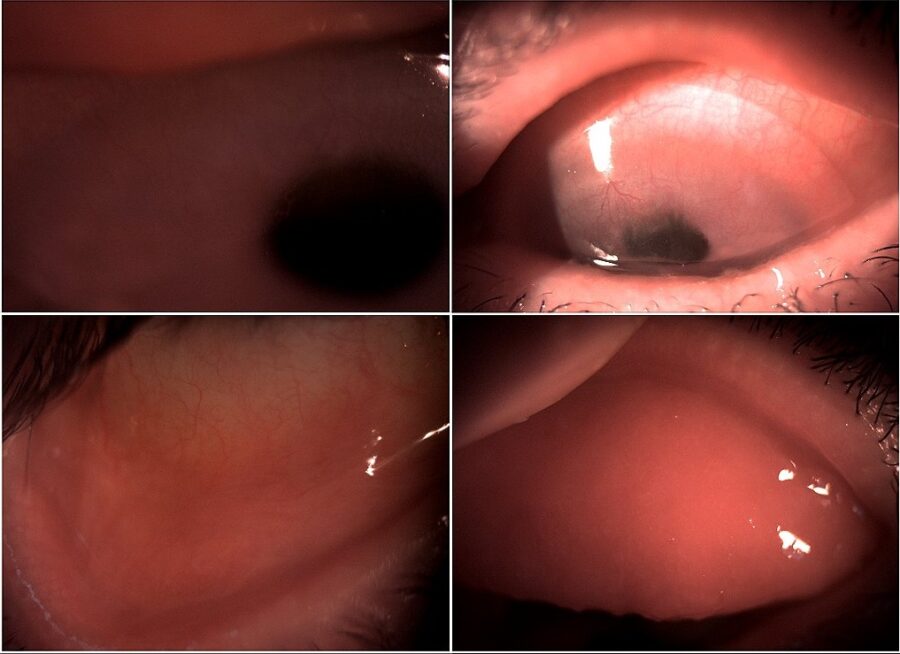
A 70-year-old Caucasian woman was referred for investigation of a relapsing bilateral ocular surface inflammation, more intense in the left eye (LE) (Figure 1). The condition had started about 2 years prior, around the time when she underwent bilateral cataract surgery with IOL implantation. She had been using ocular lubricants, as well as topical corticosteroids and antibiotics, with transient symptomatic relief but progressive visual deterioration. On the first visit, she was wearing a therapeutic contact lens on her LE, which she claimed now had only peripheral vision. Upon ophthalmological examination, her corrected distance visual acuity (CDVA) was 3/10 minus in the right eye (RE) and finger count in the LE. Biomicroscopy of the RE showed paracentral corneal thinning, superior pannus, inferior conjunctival scarring, and moderate tarsal conjunctival inflammation, while the LE featured a 360° pannus, small linear corneal epithelial ulcers, inferior conjunctival scarring, and exuberant tarsal follicles (Figure 2).
Figure 2: Slit-lamp photograph of both eyes illustrating superior pannus and inferior conjunctival scarring in the RE (left top and bottom) and pannus under a soft contact lens and exuberant tarsal follicles in the LE (right top and bottom).
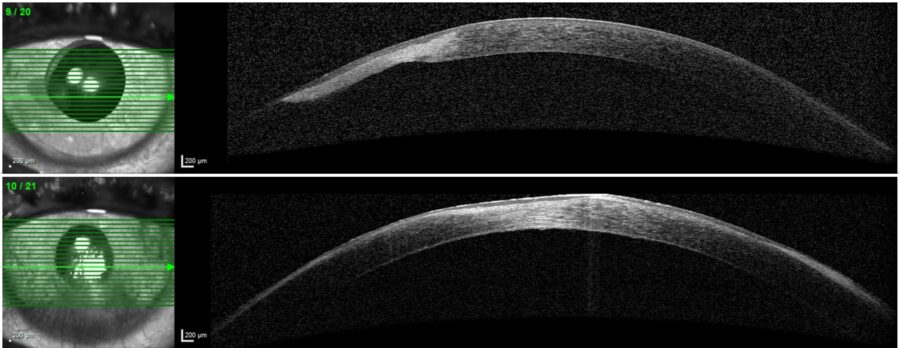
The changes in corneal thickness were registered and measured by anterior segment OCT (Figure 3).
Figure 3: Anterior segment optical coherence tomography (AS-OCT, Heidelberg Engineering®) showing bilateral corneal scarring with paracentral thinning in the RE (top) and central thinning in the LE (bottom).
The patient was then started on topical autologous serum hourly, cyclosporine 1% and ofloxacin drops, and an ocular lubricant ointment. Four weeks later, the eyes were calmer, and a bilateral conjunctival biopsy was performed. The treatment regimen was adjusted with the introduction of dexamethasone drops, tacrolimus ointment, and non-preserved artificial tears. Three months later, both symptomatic and objective improvement were noted.
Additional History
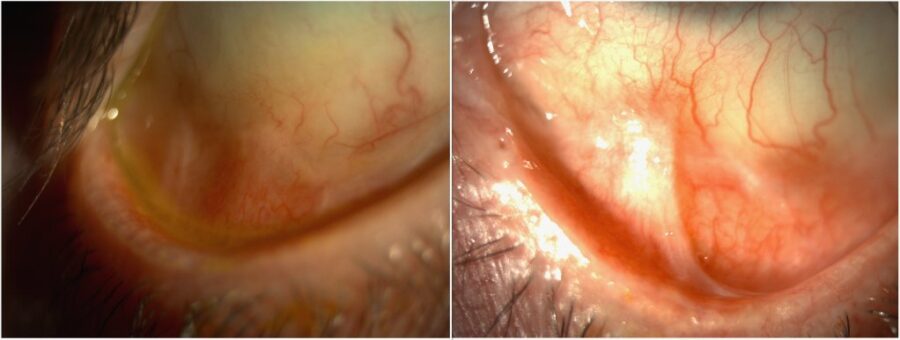
The conjunctival biopsy confirmed the clinical diagnosis of ocular mucous membrane pemphigoid stage 2. In order to achieve better long-term control and make medication weaning feasible without a rebound, immunosuppression was indicated. After a 3-month course of systemic prednisone and mycophenolate, a best-corrected visual acuity (BCVA) of 6/10 was achieved in the RE. The corticosteroid was tapered and stopped, and the immunosuppressant was maintained with good tolerability. At one-year follow-up, visual acuity remained stable and there were no signs of active ocular inflammation. The RE exhibited a paracentral scar, while the LE showed regression of the corneal neovascularization. Inferior conjunctival fibrosis and symblepharon continued unchanged in both eyes (Figure 4).
Figure 4: Slit-lamp photograph of both eyes at 1 year of immunomodulatory therapy illustrating inferior symblepharon in spite of absence of active inflammation.
Differential Diagnosis of Ocular Mucous Membrane Pemphigoid (OcMMP)
- Stevens–Johnson syndrome (SJS) / toxic epidermal necrolysis (TEN)
- Paraneoplastic pemphigus
- Graft-versus-host disease
- Mucosal-predominant epidermolysis bullosa acquisita
- Linear immunoglobulin A disease
- Dermatitis herpetiformis
- Pemphigus vulgaris
- Ocular surface squamous neoplasia (OSSN)
- Sebaceous cell carcinoma (SCC)
- Trachoma
- Adenoviral conjunctivitis
- Conjunctival trauma (chemical, thermal, surgical, or radiation-induced)
- Atopic keratoconjunctivitis
- Ocular rosacea
- Ectodermal dysplasia
Although several of the aforementioned etiologies of cicatrizing conjunctivitis have a similar clinical appearance, careful examination and history taking can aid in making the correct diagnosis. Bilaterality, other mucous or skin involvement, and progressive symblepharon are distinguishing features of OcMMP. A common confounder is medicamentosa or toxic conjunctivitis, a condition caused by prolonged eye drop administration (especially for glaucoma) that induces pseudopemphigoid. Definite diagnosis is made by conjunctival biopsies showing linear staining of the basement membrane in OcMMP.
Discussion and Literature
The term ocular cicatricial pemphigoid (OCP) has largely been replaced by ocular mucous membrane pemphigoid (OcMMP) after publication of the 2002 international consensus document. Ocular mucous membrane pemphigoid is the ocular form of mucous membrane pemphigoid (MMP), a systemic cicatrizing autoimmune disease primarily affecting orifical mucous membranes (conjunctival, nasal, oropharyngeal, genital). Ocular involvement occurs in about 70% of all MMP cases, and In some cases of MMP, esophagus, trachea, and skin may also be involved. Ocular mucous membrane pemphigoid is considered a rare disease with an estimated incidence of about 1 per 10,000 to 50,000, predominantly affecting females (2:1 over males). The age of onset is around 60 years or older and there is no racial predilection.
Ocular Mucous Membrane Pemphigoid is characterized by a chronic bilateral conjunctivitis with relapsing‑remitting periods. The underlying pathophysiological mechanism is a type 2 hypersensitivity reaction against the basal epithelial membrane of the conjunctiva in which environmental factors and genetic susceptibility are each believed to play a role. There is some evidence of association with HLA-DR4 and HLA-DQ7 positivity. The immunopathological progression of OcMMP has three distinct phases: injury, acute inflammation and proliferation, and fibrosis. The trigger for the onset of the OcMMP is unknown. It has been suggested that it may arise as a secondary pathology to acute inflammatory events or chronic inflammatory states of the conjunctiva and ocular surface.
Using a clinical staging method, defining mild, moderate, and severe forms, is an essential step in management. The two grading systems for OcMMP are the Foster (based on clinical signs) – Stage 1: subconjunctival scarring and fibrosis; Stage 2: fornix shortening; Stage 3: symblepharon formation; Stage 4: ankyloblepharon and ocular surface keratinization – and the Mondino and Brown (based on inferior fornix depth loss) – Stage 1: 0% to 25% loss; Stage 2: 25% to 50% loss; Stage 3: 50% to 75% loss; Stage 4: 75% to 100% loss.
Conjunctival biopsy with direct immunofluorescence is the gold standard in confirming the diagnosis of OcMMP, but up to 40% of patients have a negative biopsy result, which may be due to biopsy technique, concurrent quiescence, or even disease burnout. Supplementation with avidin-biotin complex immunoperoxidase (ABC) can be a useful tool. In any way, a negative biopsy result should not delay treatment in the case of a clear clinical picture.
The main goal of OcMMP treatment is to stop disease progression, since without therapy 75% of cases develop visual loss due to major complications (severe dry‑eye syndrome, corneal erosions, corneal keratinization, entropion, symblepharon). The mainstream management is systemic immunosuppression, as disease activity can be suppressed with early initiation of therapy. With long‑term systemic medication, 90% of cases can be efficiently controlled. Most patients can be managed with dapsone, azathioprine, mycophenolate, methotrexate, or cyclosporine. Cyclophosphamide, biologic agents, and immunoglobulin therapy are usually reserved for recalcitrant disease and unsatisfactory response to conventional drugs. Aside from constant lubricant medication, dry-eye syndrome may require topical steroids, cyclosporine, and tacrolimus. Surgical procedures should be planned only in the quiescent phase because minor conjunctival trauma can significantly worsen the disease.
Recent data shows that OcMMP presenting with extraocular manifestations and corneal scarring has an increased risk of stage progression and vision loss. Corneal scarring and severe inflammation at baseline are associated with an increased risk of relapse. Long-term remission for OcMMP off immunomodulatory therapy (IMT) may be achieved with a particular program of stepladder IMT and withdrawal regimens. In spite of effectiveness and good tolerability, immunosuppressive agents cannot always prevent slow progression of conjunctival cicatrization in OcMMP.
Keep in mind
- Ocular mucous membrane pemphigoid is an autoimmune cicatrizing ocular surface disease in the spectrum of mucous membrane pemphigoid.
- The diagnosis of OcMMP is based on the clinical presentation and confirmed with conjunctival biopsy and immunohistochemical analysis.
- Early diagnosis and prompt initiation of systemic immunosuppressive therapy can arrest OcMMP progression and induce prolonged remission, preventing further anatomic and functional ocular surface deterioration.
References
- Branisteanu DC, Stoleriu G, Branisteanu DE, Boda D, Branisteanu CI, Maranduca MA, Moraru A, Stanca HT, Zemba M & Balta F (2020). Ocular cicatricial pemphigoid (Review). Experimental and therapeutic medicine, 20(4), 3379–3382. https://doi.org/10.3892/etm.2020.8972
- Schonberg S & Stokkermans TJ. Ocular Pemphigoid. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK526100/
- Çiftçi MD, Korkmaz İ, Palamar M, Yaman B, Eğrilmez S, Yağcı A, Akalın T & Barut Selver Ö (2023). Clinical Approach to Ocular Cicatricial Pemphigoid. Turkish journal of ophthalmology, 53(2), 79–84. https://doi.org/10.4274/tjo.galenos.2022.34683
- Georgoudis P, Sabatino F, Szentmary N, Palioura S, Fodor E, Hamada S, Scholl H & Gatzioufas Z (2019). Ocular Mucous Membrane Pemphigoid: Current State of Pathophysiology, Diagnostics and Treatment. Ophthalmology and therapy, 8(1), 5–17. https://doi.org/10.1007/s40123-019-0164-z
- Philip AM, Fernandez-Santos CC, Ramezani K, Dolinko AH, Manhapra A, Look-Why S, Chang PY, Foster CS & Anesi SD (2023). Ocular Mucus Membrane Pemphigoid: A Primary Versus Secondary Entity. Cornea, 42(3), 280–283. https://doi.org/10.1097/ICO.0000000000003056
- Fremont F, Pelissier-Suarez C, Fournié P, Porterie M, Thevenin A, Astudillo L, Paricaud K, Gualino V, Soler V & Pugnet G (2019). Clinical Characteristics and Outcomes of Ocular Cicatricial Pemphigoid: A Cohort Study and Literature Review. Cornea, 38(11), 1406–1411. https://doi.org/10.1097/ICO.0000000000002080
- You C, Ma L, Anesi SD & Stephen Foster C (2018). Long-term remission of ocular cicatricial pemphigoid off immunomodulatory therapy. European journal of ophthalmology, 28(2), 157–162. https://doi.org/10.5301/ejo.5001050
- Ruiz-Lozano RE, Colorado-Zavala MF, Ramos-Dávila EM, Quiroga-Garza ME, Azar NS, Mousa HM, Hernández-Camarena JC, Stinnett SS, Daluvoy M, Kim T, Sainz-de-la-Maza M, Hall RP 3rd, Rodriguez-Garcia A & Perez VL (2024). Ocular Mucous Membrane Pemphigoid: The Effect of Risk Factors at Presentation on Treatment Outcomes. Ophthalmology, 131(9), 1064–1075. https://doi.org/10.1016/j.ophtha.2024.02.028
- Anesi SD, Eggenschwiler L, Ferrara M, Artornsombudh P, Walsh M & Foster CS (2021). Reliability of Conjunctival Biopsy for Diagnosis of Ocular Mucous Membrane Pemphigoid: Redetermination of the Standard for Diagnosis and Outcomes of Previously Biopsy-Negative Patients. Ocular immunology and inflammation, 29(6), 1106–1113. https://doi.org/10.1080/09273948.2020.1716988
- Doycheva D, Deuter C, Blumenstock G, Stuebiger N & Zierhut M (2011). Long-term results of therapy with mycophenolate mofetil in ocular mucous membrane pemphigoid. Ocular immunology and inflammation, 19(6), 431–438. https://doi.org/10.3109/09273948.2011.624288