Presented by: Miltiadis Balidis MD, PhD, FEBOphth, ICOphth
Edited by: Penelope Burle de Politis, MD
A 59-year-old man was referred for investigation of bilateral diffuse corneal opacities.
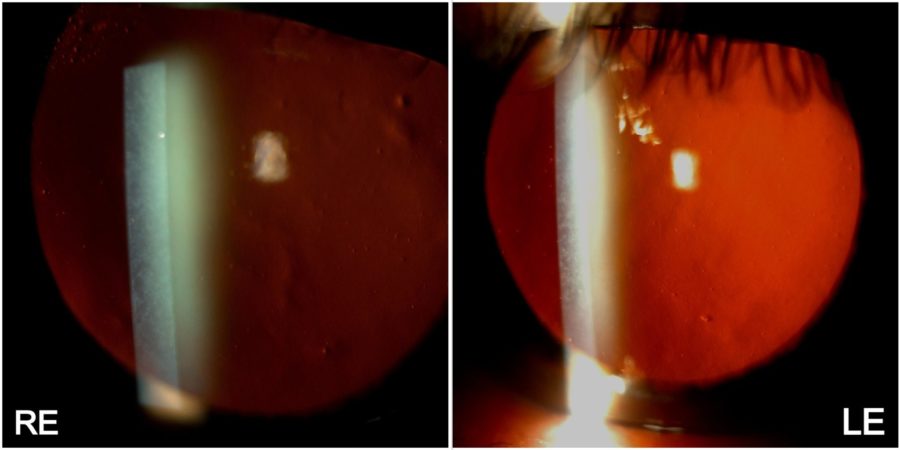
Figure 1: Low-magnification slit-lamp photograph showing a symmetrical dust-like appearance of both corneas, predominantly in the center and mid-periphery.
Case History
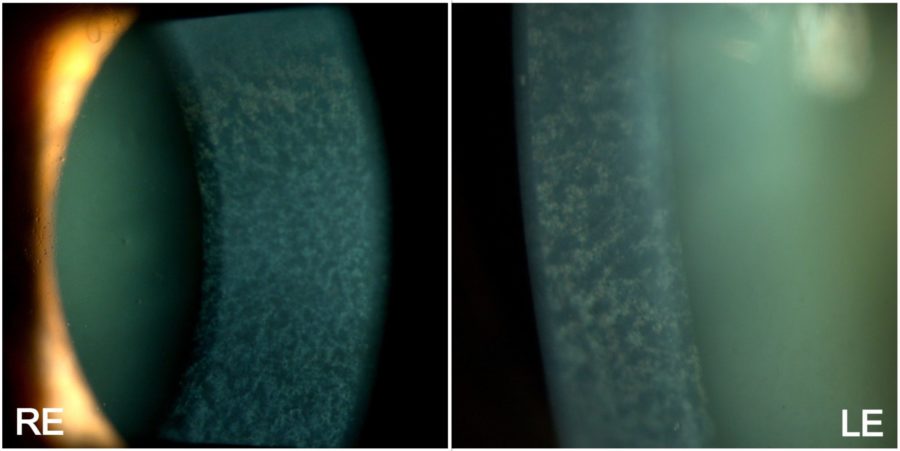
A 59-year-old Caucasian man was referred for diagnosis and management of diffuse corneal opacities of unknown duration in both eyes (BE). He had no previous records of ocular or systemic diseases. His family history was unremarkable. Upon ophthalmological examination, his corrected distance visual acuity (CDVA) was 10/10 in the right eye (RE) and 8/10 in the left eye (LE). Refractometry was -2.00 -0.75@75° in the RE and -2.00 -0.50@45° in the LE, and intraocular pressure was respectively 11 and 12 mmHg. Fundoscopy was bilaterally normal. Under biomicroscopy, BE featured a deep net of thin, confluent corneal opacities with intervening clear cornea, extending to 360° of the corneal radius, with relative sparing of the corneal periphery (Figure 1). Higher magnification revealed that the opacities varied in shape from dendritic to filiform and laid along the posterior stroma (Figure 2). The eyes were calm, and a grade 1-2 nuclear cataract was detected in the LE.
Figure 2: High-magnification slit-lamp photograph showing a normally-appearing anterior corneal stroma in both eyes, while the posterior stroma is diffusely taken by fine, pleomorphic grayish deposits. A moderately mature nuclear cataract is also noticeable in the LE.
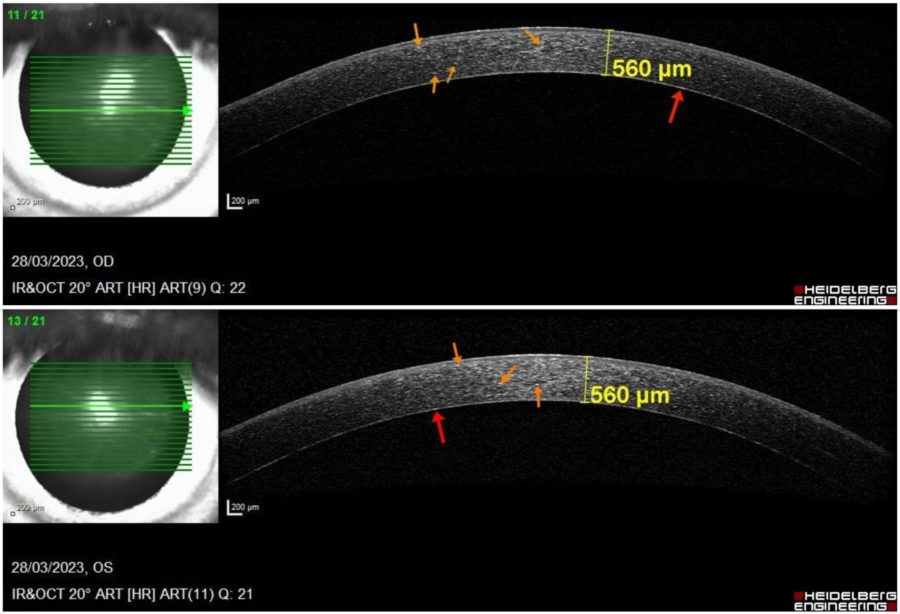
Anterior segment spectral domain optical coherence tomography (anterior SD-OCT) revealed a dense hyperreflective band in the pre-Descemet corneal stroma, evenly extending from limbus to limbus, as well as scattered hyperreflective bodies throughout the corneal stroma in BE. Corneal pachymetry was within the normal range bilaterally (Figure 3).
Figure 3: Anterior SD-OCT (Heidelberg Engineering®) displaying bilateral punctate hyperreflective particles at different depths of the cornea (orange arrows) and forming a dense band in the posterior stroma immediately anterior to the Descemet’s membrane (red arrows). There are no signs of corneal edema (central pachymetry of 560 μm in BE).
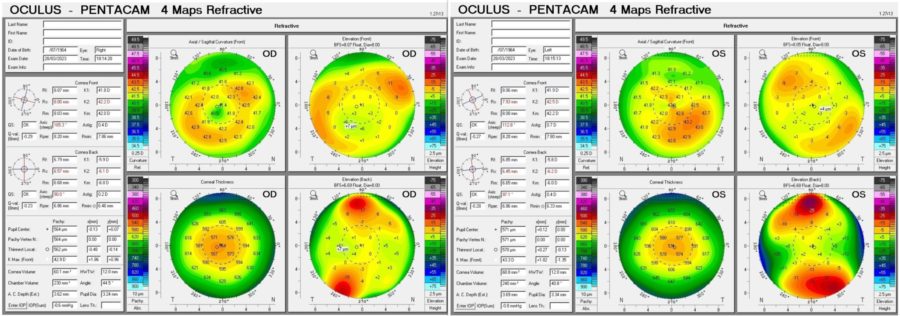
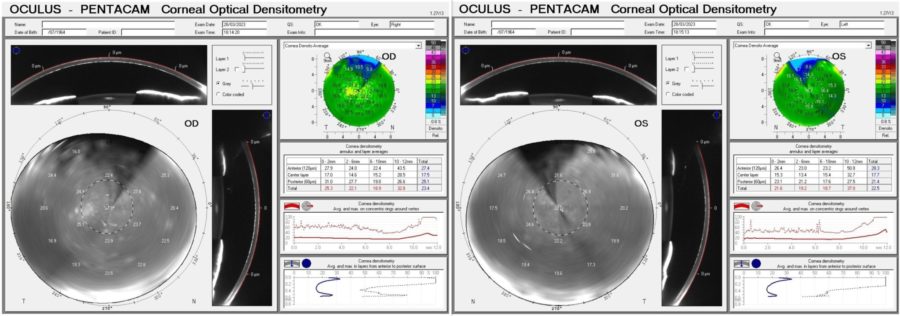
Corneal tomography displayed a slightly irregular astigmatism (Fig. 4) and Scheimpflug Corneal Densitometry was consistent with a hyperdense pre-Descemet stroma in BE (Figure 5). Keratometric values were unremarkable and the epithelial maps obtained with the Anterion® platform showed no significant changes in either eye.
Figure 4: Refractive Display of the Pentacam® corneal tomography showing slight topographic irregularity in BE. Kmax is 42.9 D in the RE and 42.9 D in the LE.
Figure 5: Corneal Optical Densitometry display (Pentacam®) demonstrating areas of increased density in all depths (anterior, center and posterior) and all circumferences (from 0-2 to 10-12 mm) of both corneas.
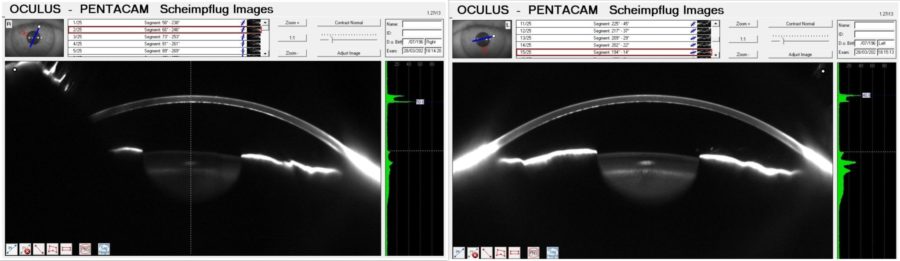
Figure 6: Scheimpflug Images (Pentacam®) revealing a bimodal peak light scattering in both corneas with a posterior peak (in gray scale units — GSU) of 50.6 in the RE and of 40.8 in the LE. Increased nuclear lens density in the LE is evident as well.
Additional History
Based on the clinical and multimodal image findings, the diagnosis of pre-Descemet corneal dystrophy was established.
Considering the lack of complaints and good visual acuity in BE, conservative follow-up was the management of choice for the moment.
Differential Diagnosis of Pre-Descemet Corneal Dystrophy
- cornea farinata
- long-term contact lens use
- Fleck corneal dystrophy
- corneal epithelial basement membrane dystrophy
- Fuchs corneal endothelial dystrophy
- crystalline keratopathy related to monoclonal gammopathy
The differentiation among the different types of posterior corneal dystrophy is based on their morphology and distribution of abnormal material within the corneal tissue. While histopathologic study remains the gold standard for diagnosis, attentive corneal examination complemented by high-resolution methods are the definite tool for accurate identification and proper management of each disorder.
Discussion and Literature
Corneal dystrophies (CDs) are a collection of hereditary non-inflammatory disorders that affect corneal transparency and refraction due to abnormal deposition of substances in the cornea. According to the International Classification of Corneal Dystrophies (IC3D — 2015), CDs are subclassified in epithelial/subepithelial, epithelial-stromal, stromal, and endothelial dystrophies, depending on their location. Patients with CD can be asymptomatic or present with bilateral visual acuity loss, typically in the form of irregular astigmatism. Symptoms can begin at various ages and treatment can range from conservative measures to corneal transplantation.
Pre-Descemet is a rarer form of CD, first described in 1947. It has been referred to as cornea farinata, posterior filiform dystrophy or posterior punctiform dystrophy, and described as the accumulation of gray-white-bluish dust-like opacities made of lipids that are characteristically located at the pre-Descemet area of the cornea. These deposits are well demarcated and can be better visualized under biomicroscopic retroillumination. Their shape can vary from circular to linear, dendritic, filiform, comma- or “boomerang”-shaped. They can be confined to the axial area of the cornea alone (axial type), be located 1 to 2 mm within the limbus, sparing both the peripheral and axial cornea (annular type), or be diffusely distributed throughout the cornea (diffuse type).
The diagnosis of PDCD is based mainly on the slit-lamp findings of fine, polymorphic opacities just anterior to Descemet’s membrane. Nonetheless, the latest high-resolution anterior segment imaging technologies allow for non-invasive assessment of corneal structure and histology. Both spectral domain optical coherence tomography (SD-OCT) and in vivo confocal microscopy (IVCM) have been used to corroborate the diagnosis of PDCD and for a better understanding of its pathophysiology. Other propaedeutic methods such as corneal tomography can be useful in discarding other corneal abnormalities that may occur simultaneously with the condition, such as keratoconus. No biological tests for PDCD are available at the moment.
Due to its nonsymptomatic nature, pre-Descemet CD (PDCD) may go undiagnosed for long. The disorder is found more often in females and usually presents later in life (third to fourth decade). The condition can be asymmetric but is always bilateral. The lesions may confluence and form clumps. Because neither the endothelium nor the epithelium are involved, there is no corneal edema, photophobia, pain, or surface changes. Corneal sensitivity is not affected.
Even though patients with pure PDCD are asymptomatic, the condition has been found in conjunction with other CDs and systemic disorders such as ichthyosis. Isolated PDCD is often sporadic; however, a genetic form of the disease, probably with autosomal dominant inheritance and unknown gene location, has been described. A genetic basis has been identified in the subtype punctiform and polychromatic pre-Descemet corneal dystrophy (PPPCD), which also features increased corneal stiffness.
Although classically described as pre-Descemet, PDCD may involve the entire cornea. Both transmission electron microscopy and IVCM have evidentiated enlarged hyperreflective posterior stromal keratocytes with either hyperreflective or hyporeflective intracellular dots corresponding to intracytoplasmic inclusions of electron-dense, lipofuscin-like lipoprotein. This degenerative change in posterior stromal keratocytes is likely to be the origin of the slit-lamp presentation of PDCD. On the other hand, IVCM allows for a layer-by-layer analysis and demonstrates that the pathology is more extensive, with signs not only in the corneal stroma, but also in the endothelium and sub-basal nerves, whilst without functional compromise.
The current trend is that PDCD may be a variant of different corneal diseases sharing the same pathophysiological pathway. Latest evidence suggests that sporadic PDCD belongs in the spectrum of degenerative corneal disorders. Similar histological findings have been described in other conditions such as long-term contact lens wear. Recently, an identical corneal disorder was described in dogs, both through OCT and IVCM, with signs that support the degenerative theory.
For the moment, PDCD is neither a contraindication for laser corneal ablation nor an indication for phototherapeutic corneal procedures, as uneventful outcomes of PRK have been reported with no alteration of the corneal deposits. Conservative follow-up remains the management of choice for isolated cases. Genetic counseling is advised in cases where a genetic form can be traced back in the family tree.
Keep in mind
- Unlike other forms of posterior corneal dystrophies, PDCD is predominantly located at the pre-Descemet stroma, thus its absence of symptoms and lack of visual impairment.
- Even though the diagnosis of PDCD is typically obtained through biomicroscopy, other non-invasive imaging methods can be used to confirm it, exclude other corneal disorders, and help understand its physiopathology.
- Due to its benign clinical course, PDCD does not require any intervention. However, until the condition is fully elucidated, regular follow-up is advised.
References
- Moshirfar M, Bennett P & Ronquillo Y (2022). Corneal Dystrophy. In StatPearls. StatPearls Publishing.
- Vemuganti GK, Rathi VM & Murthy SI (2011). Histological landmarks in corneal dystrophy: pathology of corneal dystrophies. Developments in ophthalmology, 48, 24–50. https://doi.org/10.1159/000324261
- Grayson M & Wilbrandt H (1967). Pre-Descemet dystrophy. American journal of ophthalmology, 64(2), 276–282. https://doi.org/10.1016/0002-9394(67)92524-x
- Curran RE, Kenyon KR & Green WR (1974). Pre-Descemet’s membrane corneal dystrophy. American journal of ophthalmology, 77(5), 711–716. https://doi.org/10.1016/0002-9394(74)90536-4
- Boere PM, Bonnet C, Frausto RF, Fung SSM & Aldave AJ (2020). Multimodal Imaging of Pre-Descemet Corneal Dystrophy Associated With X-Linked Ichthyosis and Deletion of the STS Gene. Cornea, 39(11), 1442–1445. https://doi.org/10.1097/ICO.0000000000002382
- Alió Del Barrio JL, Chung DD, Al-Shymali O, Barrington A, Jatavallabhula K, Swamy VS, Yébana P, Angélica Henríquez-Recine M, Boto-de-Los-Bueis A, Alió JL & Aldave AJ (2020). Punctiform and Polychromatic Pre-Descemet Corneal Dystrophy: Clinical Evaluation and Identification of the Genetic Basis. American journal of ophthalmology, 212, 88–97. https://doi.org/10.1016/j.ajo.2019.11.024
- Alafaleq M, Georgeon C, Grieve K & Borderie VM (2020). Multimodal imaging of pre-Descemet corneal dystrophy. European journal of ophthalmology, 30(5), 908–916. https://doi.org/10.1177/1120672119862505
- Malhotra C, Jain AK, Dwivedi S, Chakma P, Rohilla V & Sachdeva K (2015). Characteristics of Pre-Descemet Membrane Corneal Dystrophy by Three Different Imaging Modalities-In Vivo Confocal Microscopy, Anterior Segment Optical Coherence Tomography, and Scheimpflug Corneal Densitometry Analysis. Cornea, 34(7), 829–832. https://doi.org/10.1097/ICO.0000000000000454
- Park S, Sebbag L, Moore BA, Casanova MI, Leonard BC, Daley NL, Steele KA, Li JY, Murphy CJ & Thomasy SM (2022). Multimodal ocular imaging of known and novel corneal stromal disorders in dogs. BMC veterinary research, 18(1), 117. https://doi.org/10.1186/s12917-022-03214-7
- Kontadakis GA, Kymionis GD, Kankariya VP, Papadiamantis AG & Pallikaris AI (2014). Corneal confocal microscopy findings in sporadic cases of pre-descemet corneal dystrophy. Eye & contact lens, 40(2), e8–e12. https://doi.org/10.1097/ICL.0b013e318273be9f