Drug Des Devel Ther
2024 Nov 25:18:5367-5375
PMID: 39624769
PMCID: PMC11609416
DOI: 10.2147/DDDT.S477677
Affiliations
1Vitreoretinal Department, Ophthalmica Eye Institute, Thessaloniki, Greece.
2Department of Ophthalmology, School of Medicine, University of Patras, Patras, Greece.
3Division of Ophthalmology and Visual Sciences, School of Medicine, University of Nottingham, Nottingham, UK.
4Department of Ophthalmology, Queen’s Medical Centre, Nottingham University Hospitals, Nottingham, UK.
5First Department of Ophthalmology, AHEPA University Hospital, School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Abstract
Purpose: To evaluate the efficacy of the dexamethasone implant on the electrophysiological profile of Diabetic Macular Oedema (DMO) patients over six months.
Methods: In this prospective, single-center study 30 eyes of 22 patients were examined using comprehensive baseline assessments including best-corrected visual acuity (BCVA), central retinal thickness (CRT), contrast sensitivity (CS) and multifocal electroretinogram (mfERG), before and after 0.7mg dexamethasone implant injection, with follow-ups at months 1, 2, 4, and 6. The study employed mixed models to analyse within-subject and between-subject correlations, considering the complexities of multiple measurements per subject.
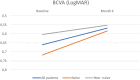
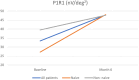
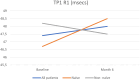
Results: At baseline, BCVA was 0.66 ± 0.104 logMAR, improving to 0.568 ± 0.104 logMAR by month 6 (P > 0.05). CRT significantly reduced from 521 ± 28.7 μm to 336 ± 28.7 μm (P < 0.05). CS slightly increased from 26.8 ± 1.23 letters to 28.5 ± 1.05 letters (P > 0.05). P wave amplitude saw a notable rise from 33.4 ± 5.66 μV to 47.9 ± 5.43 μV (P < 0.05). P wave implicit time changed minimally from 47.4 ± 0.503 seconds to 48.0 ± 0.503 seconds (P > 0.05). No severe adverse events were recorded.
Conclusion: These results underscore the 0.7mg dexamethasone implant’s potential in improving certain electrophysiological markers in DMO, while also highlighting the need for further investigation into its comprehensive impact on retinal function.
Keywords: contrast sensitivity; dexamethasone implant; diabetic macular oedema; electroretinogram; macular thickness; visual acuity; visual function.
© 2024 Tranos et al.
Conflict of interest statement
The authors have no other conflict of interest to declare.
References
-
- Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39(9):1643–1649. doi:10.2337/dc15-2171 – DOI – PubMed
- Romero-Aroca P. Targeting the pathophysiology of diabetic macular edema. Diabetes Care. 2010;33(11):2484–2485. doi:10.2337/dc10-1580 – DOI – PMC – PubMed
- Browning DJ, Stewart MW, Lee C. Diabetic macular edema: evidence-based management. Indian J Ophthalmol. 2018;66(12):1736–1750. doi:10.4103/ijo.IJO_1240_18 – DOI – PMC – PubMed
- Panos GD. Evolution of intravitreal therapy for retinal and macular disorders. J Int Med Res. 2020;48(1):300060518771411. doi:10.1177/0300060518771411 – DOI – PMC – PubMed
- Grover D, Li TJ, Chong CC, Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2008;1:CD005656. doi:10.1002/14651858.CD005656.pub2 – DOI – PMC – PubMed
- Schwartz SG, Scott IU, Stewart MW, Flynn Jr. HW Jr. Update on corticosteroids for diabetic macular edema. Clin Ophthalmol. 2016;10:1723–1730. doi:10.2147/OPTH.S115546 – DOI – PMC – PubMed
- Baget-Bernaldiz M, Romero-Aroca P, Bautista-Perez A, Mercado J. Multifocal electroretinography changes at the 1-year follow-up in a cohort of diabetic macular edema patients treated with ranibizumab. Doc Ophthalmol. 2017;135(2):85–96. doi:10.1007/s10633-017-9601-2 – DOI – PMC – PubMed
- Mastropasqua R, Toto L, Borrelli E, et al. Morphology and function over a one-year follow up period after intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema. PLoS One. 2015;10(12):e0145663. doi:10.1371/journal.pone.0145663 – DOI – PMC – PubMed
- Creel DJ. Electroretinograms. Handb Clin Neurol. 2019;160:481–493. – PubMed
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–1806. doi:10.1001/archopht.1985.01050120030015 – DOI – PubMed
- Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62(4):231–236. doi:10.1159/000499540 – DOI – PubMed
- Bearse MA Jr, Ozawa GY. Multifocal electroretinography in diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2014;14(9):526. doi:10.1007/s11892-014-0526-9 – DOI – PubMed
- Bearse MA Jr, Han Y, Schneck ME, Adams AJ. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2004;45(1):296–304. doi:10.1167/iovs.03-0424 – DOI – PubMed
- Tonade D, Kern TS. Photoreceptor cells and RPE contribute to the development of diabetic retinopathy. Prog Retin Eye Res. 2021;83:100919. doi:10.1016/j.preteyeres.2020.100919 – DOI – PMC – PubMed
- Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19(6):1816. – PMC – PubMed